Dating Techniques
The Earth is 4.5 billion years old, but how do we know this? Many may be familiar with radiocarbon dating being used to date fossils and other organic materials. However, this only works for things up to 60,000 years old due to 10 half-lives passing, leaving very little radioactive carbon in the sample. For much older materials, a method known as Uranium-Lead dating (a form of radiometric dating) is applied to find their age. When igneous rocks are first formed from cooled magma, a trace mineral called zircon is sometimes formed, containing small amounts of uranium that decay over time. This can be used to date rocks from 1 million to 4.5 billion years ago.
Uranium-Lead Dating
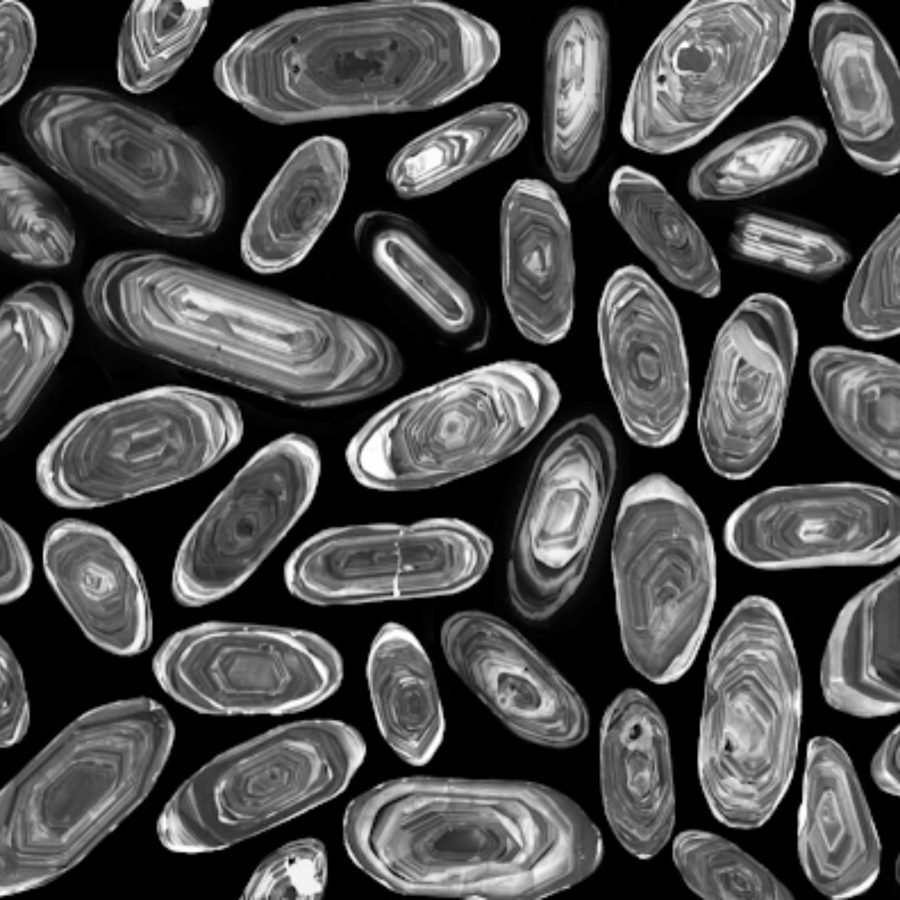
Zircon (ZrSiO4 ) forms in crystals around 1mm in size and contains small amounts of uranium isotopes that undergo radioactive decay to lead. As the zircon crystal heavily rejects lead as it forms, all lead found in the crystal must have come from the decay of uranium (radiogenic). Zircon is also extremely durable and resistant to chemical erosion, meaning the chemical characteristics at its formation are preserved. This makes it the ideal mineral for the job; however, other crystals such as monazite can also be used.
Two different decay routes can be traced: Uranium 238 to lead 206 with a half-life of 4.5 billion years and uranium 235 to lead 207 with a half-life of 710 million years.
Half-life is the time it takes for half of the radioactive nuclei to decay. This number is fixed for each isotope of an element, so the ratio of uranium to lead in a sample can be used to date it accurately, with an estimated only 0.1-1% error.
The oldest zircon crystals ever dated were found in the Jack Hills, Western Australia, and were found to be 4.04 billion years old, making them the oldest minerals dated so far. However, the Murchison meteorite is thought to have been made from grains 7 billion years old: much older than our solar system!
This article was written by Sebi Ivimey.